
Ovulation Induction using Clomiphene Citrate
What is clomiphene citrate?
Clomiphene citrate is one of the most widely used drugs in fertility treatment. It is taken as a tablet and is used to induce ovulation in women who do not ovulate regularly or not at all (e.g. those with polycystic ovarian syndrome).
How does it work?
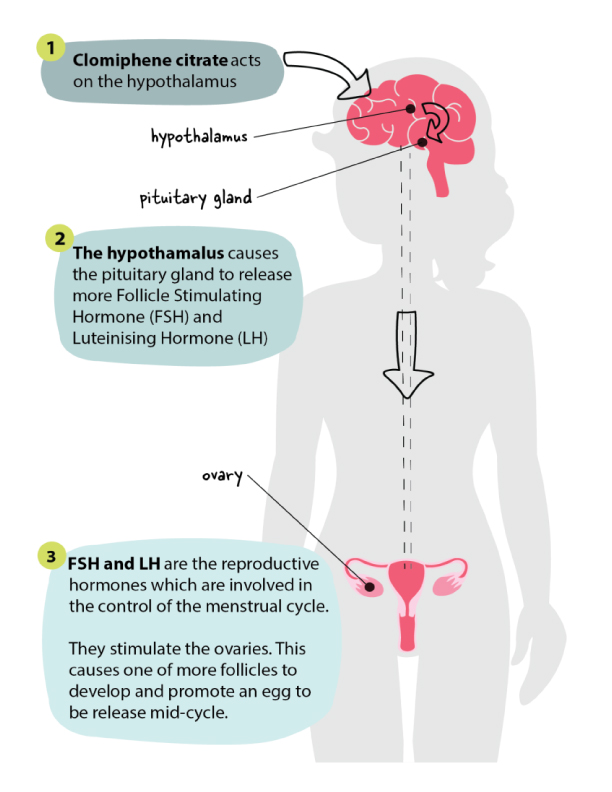
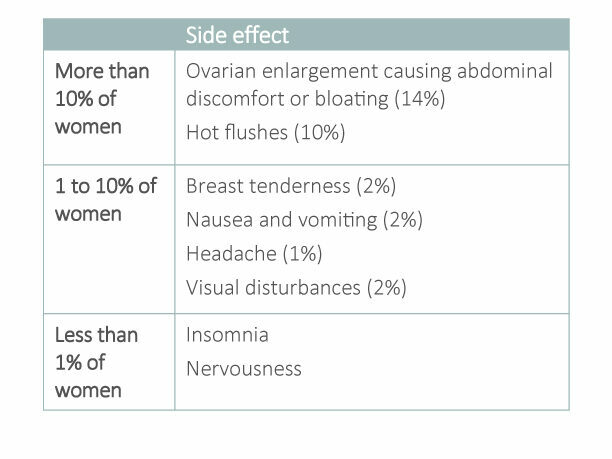
Are there any side effects?
If you do experience side effects, they are usually mild and quickly improve soon after stopping therapy. Let the fertility clinic know if you have any symptoms you are worried about.
The most commonly reported side effects with clomiphene citrate are:
The centre for Adverse Reactions Monitoring (CARM) have previously received a report of ischaemic stroke in a patient taking clomiphene. The patient was not reported to be taking any other medicines at the time of the stroke. Patients should be advised to stop taking clomiphene and get emergency treatment if they experience numbness, weakness or paralysis on one side of the body, slurred speech, sudden blurred vision, confusion or unsteadiness – these could be signs of stroke1.
Is there a risk of multiple pregnancies
with clomiphene?
Multiple pregnancies (more than one fetus) can occur using any fertility drug. Multiple pregnancies may be harmful for both mother and the foetuses.
With natural conception, one in every 80 births will be a multiple pregnancy. With clomiphene, the risk of twins is about 7 to 9%, and of triplets about 0.3%.
To reduce risk, it is especially important that you have the blood tests when advised during your clomiphene cycle.
There is no evidence that clomiphene increases the risk of miscarriage or causes any congenital abnormalities in offspring. Similarly, short term use of clomiphene is not believed to be associated with any increased risk of cancer or other health problems in women taking this drug.
Starting your clomiphene cycle
If you do not get periods very frequently you may be asked to do some initial blood tests and/or take a course of Provera to bring on a withdrawal bleed. Blood test forms will be mailed to you or given to you at your consultation.
Please note: your first cycle will require higher levels monitoring (see below).

Additional notes
Changing doses
Usually for your first cycle the clomiphene dose will be 50mg/day. However, women differ in how they respond to clomiphene.
Because of this, your dose may be increased (or occasionally decreased) in 25-50mg amounts across cycles. The results of your monitoring will let us know if we need to change your dose.
The doctor will review your results
at the end of your cycle.
Blood tests
All blood tests are carried out at Labtests. We prefer you to do blood tests first thing in the morning using the blood form you will be given, as this allows us to receive the result that afternoon.
- 80% of women ovulate in response to clomiphene.
- 40% will conceive on clomiphene.
- 15-20% of women are “resistant” to clomiphene i.e. they fail to ovulate in response to increasing doses of clomiphene.
For these reasons, it is important that you have regular reviews every 3-4 months with your doctor. This will help us know if you should keep going with clomiphene therapy or if any additional tests are needed.
Costs
Ovulation induction with clomiphene is not a publicly funded treatment. The cost of a cycle is $440.
Charges cover the cost of blood tests, and scan if required and interpretation of results.
Clomiphene is a prescription cost only.
You will be invoiced for your treatment when you phone in on your Day 1.
Reference:
1Medsafe. New Zealand Medicines and Medical Devices Safety Authority. Ministry of Health. 2 Sept 2015